
Batteries
Many years of experience and Bio-based applications enabled Jongia to propel in the new and fast growing business area of the Energy market. Jongia’s stirring and mixing equipment comply with extreme criteria concerning emission values, shaft alignment tolerances and rotational accuracy.
Battery Chemicals with Jongia Mixing Technology!
Battery chemicals can be grouped under three main categories,- electrolytes for secondary lithium-ion batteries,
- electrolytes for primary lithium batteries,
- electrolytes for super-capacitors.
The newest energy markets require Mixing Technology at the lowest power consumption

Electroclyte for one million electric cars
Applications
Batteries explained
The chemistry of a battery

Frequently Asked Questions
What are the main categories of battery chemicals?
Battery chemicals can be grouped into three main categories: electrolytes for secondary lithium-ion batteries, electrolytes for primary lithium batteries, and electrolytes for super-capacitors. Each category plays a crucial role in the performance and efficiency of different battery types.
What is the purpose of the electrolyte in lithium-ion batteries?
The electrolyte in lithium-ion batteries, composed of a solvent and lithium salt, is vital for transporting lithium ions. It acts as the “blood” of the battery, ensuring high voltage and specific energy, which contributes to the battery’s overall performance and advantages.
What role does Jongia play in battery production?
Jongia supplies bespoke agitators for the electrolyte production process in a new factory in Poland. Our equipment is designed to meet the unique needs of electrolyte production, reinforcing Jongia’s reputation as a key player in the battery production market.
What are the components of a battery?
A battery typically consists of three parts: the positive electrode (anode), negative electrode (cathode), and electrolyte solution. These components work together to facilitate the flow of electricity when connected to a device.
How are lithium-ion batteries expected to evolve in the future?
Lithium-ion batteries are projected to dominate energy storage solutions, with global production set to increase significantly. Advancements in technology and growing demand for electric vehicles and renewable energy will drive this growth, making batteries more efficient and cost-effective.
Energy – Contacts

Tom Pruymboom
Sales Director
Area Worldwide

Jan Siert Tjeerdsma
Project manager
Technical Specialist
Energy – Related Articles

Is Sodium-Ion the next generation sustainable Battery?
The sodium-ion battery (NIB or SIB) is a type of rechargeable battery that uses sodium ions (Na+) as its charge carriers. Its working principle and cell construction are almost identical with those of lithium-ion battery (LIB) types, but replace lithium with sodium.

What is the recycling process for lithium
Current commercial lithium ion batteries mainly contain transition metal oxides or phosphates, aluminum, copper, graphite, organic electrolytes containing harmful lithium salts, and other chemicals. Therefore, the recycling and reuse of spent lithium ion batteries has been paid more and more
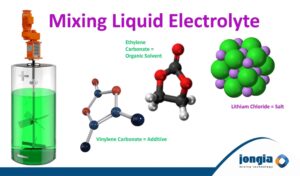
Mixing Electrolyte for Ion-Lithium Batteries
Electrolyte as basis for Ion-Lithium Batteries plays a key role in transporting the positive lithium ions between the cathode and anode, and consequently the charging and discharging performance of the battery. Hence, it needs to be checked for potential impurities.








